Jute Bleaching Process Explained in Easy Words
Introduction
Jute is a natural bast fiber. It is widely used for packaging, home textiles, and fashion. The jute bleaching process is an important process before dyeing or finishing jute. Impurities and natural color are removed from jute by using the jute bleaching process. The bleaching process of jute helps make the fabric lighter, cleaner, and suitable for bright shades. However, this article is about the jute bleaching process and the calculation of the jute bleaching recipe.
Why is bleaching important for jute?
Jute fiber is now used for making sustainable, eco-friendly products. So, the jute fiber must be free of contamination. Generally, raw jute contains natural gums, lignin, oil, and natural pigments. These impurities are the main cause of uneven dyeing. But the bleaching process removes these unwanted materials. Hence, jute fiber becomes softer and lighter in color. The jute bleaching process also improves the fabric’s absorbency and overall appearance.
You May Read: Eco-Friendly Denim Re-Dyeing Process with Charcoal
Common Bleaching Agents used for Jute Bleaching
Bleaching agents break down natural coloring matter from jute fiber.The following common chemicals are used for jute bleaching :
- Hydrogen Peroxide (H2O2): Hydrogen peroxide is used widely. Because it gives a cleaner shade. Besides, it less damage the fiber.
- Sodium Hypochlorite (NaOCl): Sodium hypochlorite is used effectively. But it can weaken jute fibers if not properly controlled.
- Sodium chlorite (NaClO2): Sodium Chlorite is used in some industries for high whiteness.
- Peracetic Acid (PAA): Peracetic acid is used as an alternative of bleaching agent. It is a modern, eco-friendly bleaching agent. It is used for a sustainable textile process.
You May Read: Jute Scouring Process: A Complete Easy Guide for Beginner
Typical Recipe for Jute Bleaching Process
| Chemical | Amount (gm/l) |
| Wetting agent (TRO) | 1.0-2.0 |
| Detergent | 0.5-1.0 |
| Na-silicate | 6.0-10.0 |
| Caustic soda | 0.5-1.0 |
| Soda ash | 2.0-4.0 |
| Hydrogen peroxide 50% | 4.0-6.0 |
| Temperature | 85-900 C |
| Time | 40-60 |
| pH | 10.5-11 |
| M:L | 1:30 |
| Material weight | 10 gm |
Recipe Calculation
Recipe calculation following formulas is used for jute bleaching process
- Required auxiliary= (Total amount of liquor× recipe amount) ÷ (1000× stock solution)
Now, let’s calculate the recipe of jute bleaching
- Total amount of liquor required for cotton pigment dyeing process= 10×30= 300 ml
- Wetting agent required= {total amount of liquor× recipe amount (gm/l)} ÷ (1000×stock solution) = (300×2) ÷ (1000× 2%) = 30 ml [ use 2% stock solution]
- Detergent required= (300×1) ÷ (1000× 2%) = 15 ml [ use 2% stock solution]
- Na-silicate required= (300×6) ÷ (1000× 2%) = 90 ml [ use 2% stock solution]
- Caustic soda required= (300×1) ÷ (1000× 2%) = 15 ml [ use 2% stock solution]
- Soda ash required= (300×2) ÷ (1000× 2%) = 30 ml [ use 2% stock solution]
- Hydrogen peroxide required= (300×4) ÷ (1000× 2%) = 60 ml [ use 2% stock solution]
- Required water= {300- (30+15+90+15+30+60)}= 60 ml
Jute Bleaching Process Curve
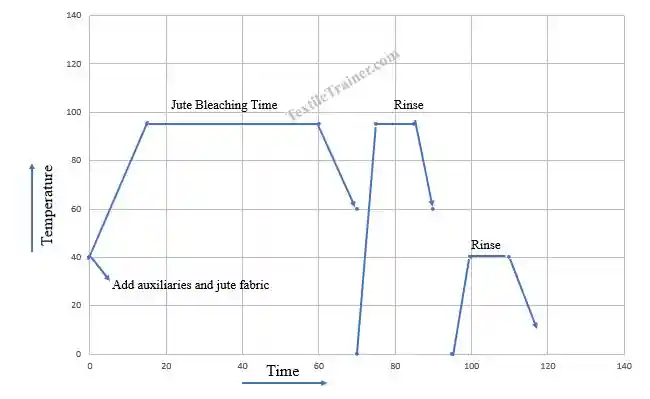
Jute Bleaching Procedure
- At first, all auxiliary, stabilizer, and caustic soda are added to the pot. Now, pH is checked, and ensure it is 10.5-11.
- Now, the hydrogen peroxide and the jute fabric are added to the pot.
- Subsequently, run the machine and raise the temperature to 85-900 C .
- The next step, the machine is run for 40-60 minutes.
- After 40-60 minutes, the machine temperature is cooled down the temperature to 70°C and the drop the bath .
- Now, the jute fabric is rinsed twice with hot and cold water, respectively.
- Finally, jute fabric is treated with a peroxide-killing agent. Peroxide killing agent help to remove residual peroxide.
Bleached Jute Fabric

Precautions during jute bleaching
- Always maintain a pH between 9-11 to prevent fiber damage.
- Avoid excessive temperature or bleaching time.
- After bleaching, it is crucial to properly wash and neutralize the jute fabric to prevent a yellowish shade after drying.
Conclusion
The bleaching process is a crucial step in preparing jute for the subsequent process. A well-bleaching process helps jute look cleaner and absorbs color. If you have any questions about the jute bleaching process, please let me know in the comments below.
FAQs
- Why is the bleaching process important for jute fabric?
- The bleaching process is crucial for jute fabric. Because the bleaching process removes natural impurities such as lignin, wax, and oils, and natural color. It also helps to improve the whiteness, absorbency, and dye uptake of jute fabric.
- Which chemical is commonly used for bleaching jute?
- Hydrogen peroxide is the most common bleaching agent for jute. Hydrogen peroxide helps to achieve good whiteness without damaging the fiber.
- What is the role of a stabilizer in jute bleaching?
- Stabilizer plays an important role in the jute bleaching process. Especially when hydrogen peroxide is used. Stabilizer controls the breakdown of hydrogen peroxide. Besides, it helps to protect the fiber from damage.
- What happens if the pH is too high during the bleaching process?
- Peroxide decomposes it pH is high. Additionally, high pH damages the fabric. The ideal pH range for safe bleaching is 9-10.
- Is hydrogen peroxide bleaching eco-friendly?
- Hydrogen peroxide bleaching is not considered as an eco-friendly bleaching agent. Because hydrogen peroxide has harmful effects on the environment and labor health. Peracetic acid serves as an alternative to traditional bleaching agents.
Reference
- S. N. Chattopadhyay, K. K. Samanta, L. Ammayappan, R. K. Ghosh, N. Kumar, and A. Khan, “Reclaiming peracetic acid bleach baths for sequential bleaching of lignocellulosic fibres,” vol. 50, no. June, pp. 150–159, 2025, doi: 10.56042/ijftr.v50i2.8135.
- Hossain, M. F. (2015). Practice of Textile Coloration, Volume-I. Dhaka: Books Fair Publications.
- Hossain, M. S. (2014). Introduction to Textile Engineering. Dhaka: Books Fair Publications.



