Magic Dye (Ice color): Azoic Dyeing of Cotton in Easy 3 Steps
Introduction
This article is about the azoic dyeing of cotton. Azoic is also called a magic dye. Unlike reactive or direct dyes, azoic colors are not ready-made dyes. It is an insoluble dye. With the help of the coupling of two individual components, the insoluble azoic colors are synthesized inside the fiber. An aromatic diazonium salt and an aromatic hydroxy compound are these two components.
Why is azoic dye called “magic dye” and “Ice color”?
In the azoic dyeing of cotton process, initially, cotton fabric is treated with naphthol (a colorless compound). Subsequently, cotton fabric passed through the diazo solution. When the diazonium compound reacts with the naphthol inside of the cotton fabric, a bright color appears magically within seconds. Thus, azoic dye is familiar among dryers and textile workers as a magic dye. On the other hand, the diazotization step needs a very low temperature, about 0-5°C. Low temperature keeps the diazo compound stable. To maintain the low temperature, crushed ice is used in the bath. Because of this dependency on ice for azoic dyeing of cotton, it is also called ice color in the textile industry.
Feature of Azoic dye
- Azoic dyes contain an azo group (-N=N-) in their chemical structure. Hence, these dye is called azoic dye.
- This dyestuff has good lightfastness properties.
- Basically, azoic dye used for dyeing cotton and other cellulosic fiber.
- This dyestuff has poor to good alkali resistance.
- It is suitable for light shade dyeing.
- Naphthol is dispersed in alcohol as well as in Turkey RED(T.R.) Oil.
- Azoic dye brightness is good.
- Wash fastness properties of azoic dye are good.
Azoic Dye chemistry
Following are the azoic dye chemistry. Azoic dye chemistry can be divided into three steps. The three steps of azoic dye chemistry are:
- Naphtholation
- Diazotization
- Coupling/Developing
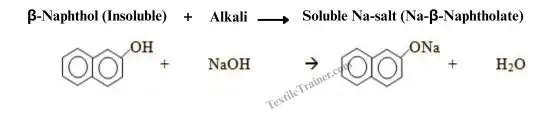
1. Naphtholation
Naphthols don’t dissolve in water, but they can be made water soluble by treating them with alkali. Alkali reacts with naphthol to form a sodium naphtholate salt. Sodium naphtholate salt is soluble in water. This allows the naphthol to penetrate the cotton fibers.
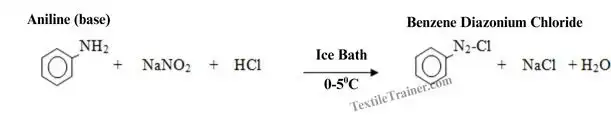
2. Diazotization
In this step, a base with an amino group (-NH) reacts with sodium nitrite (NaNO2) in the presence of hydrochloric acid (HCl) and produce diazonium chloride. But, temperature should low (0-50C).
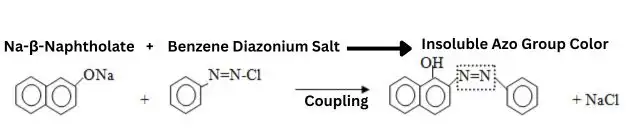
3. Coupling/ Developing
Now, the naphthol-treated material is placed in a bath containing a diazonium solution. In this bath, coupling reaction takes place. Instant, colors are form in the fabric magically. But, it crucial to maintain correct pH (3 to 6).
Recipe for Azoic dyeing of Cotton
Naphtholation Recipe
- Naphthol= 2.5-3% (On weight of fabric)
- T.R oil= 1 gm/l
- Caustic soda= 5-6 gm/l
- Common salt= 10-20 gm/l
- Time= 5 min ( Naphtholation reaction takes place with in 30 see to 1 min)
- Temperature= Room temperature
- M:L= 1:40
- Sample weight= 5 gm
Diazotization Recipe
- Base color= 4% (On weight of fabric)
- NaNO2= 2% (On weight of fabric)
- Acetic acid=2% (On weight of fabric)
- Sodium acetate=1% (On weight of fabric)
- HCl=3% (On weight of fabric)
- Temperature = 0-50C (with ice)
- Time= 20-25 min
Aftertreatment Recipe
- Soap= 2-3 gm/l
- Soda ash=1-2 gm/l
- Temperature= 60-900 C
- Time= 10-15 min
- M:L= 1:40
Azoic Dyeing Recipe Calculation
| Naphtholation | Diazotization |
| Amount of water= 200 ml | Amount of water= 200 ml |
| Amount of naphthol= 0.15 gm | Amount of base color= 0.2 gm |
| Amount of T.R oil= 20 ml (1%stock solution) | NaNO2= 10 ml (1%stock solution) |
| Caustic soda= 120 ml (1%stock solution) | Acetic acid= 10 ml (1%stock solution) |
| Common salt= 4 gm | Sodium acetate= 5 ml (1%stock solution) |
| HCl= 15 ml (1%stock solution) |
You can read: Easy Dyeing Recipe Calculation Formula with Proper Example
Dyeing process of cotton fabric with azoic dye
Azoic dyeing of cotton fabric requires 3 steps. The following are the 3 steps involved in azoic dyeing process of cotton fabric.
Step-1: Naphtholatoin ( Preparation of naphthal solution)
Initially, wetting agent (T.R. oil) mixed with naphthol paste. Next step, caustic soda solution add and boil it to prepare a clear solution. Now, common salt added to accelerate the process. Finally, dissoluble naphthol convert into soluble naphthol. Then, the fabric is immersed into naphthol solution. This process is completed at room temperature with in 30 sec to 1 minute.
Step-2: Diazotization (preparation of fast base solution)
Firstly, base paste is prepared by using warm water and sodium nitrate. Subsequently, HCl is added and stir the solution with ice piece to maintain the low temperature (0-50 C). Sodium acetate and acetic acid are added after 20 minutes. Common salt (10-20 gm/l) can be used to achieve good result.
Step-3: Coupling (Development process)
Now, the naphtholated fabric ( prepared in step-1) squeezed to remove excess naphthol solution(50%). Otherwise, development reaction may take place with the naphthol outside of the fiber. As a result, color will wastage. However, naphtholated fabric are soak into the fast base solution ( prepared in step-2). Magically color is developed in the fabric within short time.
Finally, fabric is squeezed and wash with cool water. Then, fabric is treated with HCl (1-2 gm/l) . subsequently, fabric is treated according above mentioned aftertreatment recipe. If necessary treat with acetic acid to neutralize the residual alkalis.

FAQ
Why is azoic dye called “magical dye”?
Initially, azoic dye is colorless dye. But, the color is developed after coupling step magically. Thus, azoic dyed is called “magical dye”
Why is azoic dye also called “ice color”?
Azoic dyeing of cotton is divided into three step (Naphtholation, diazotization, and coupling). In diazotization step, fast base solution is prepared. In this step, the reaction must be kept at a low temperature (0-50C) to keep the diazonium salt stable. To main the low temperature, crushed ice is used. Hence, azoic dye is also called “ice color”.
Is azoic dyeing eco-friendly?
Not fully. Because, in azoic dyeing process different chemical is used which is harmful for environment. So, proper effluent treatment are needed. Thus, azoic dyeing is not eco-friendly.
What types of fibers can be dyed with azoic dyes?
Azoic dyes are used mainly for cellulosic fibers e.g. cotton, linen, and viscose.
Can azoic dyeing be done at home?
Yes. But it is not recommended. Because, in azoic dyeing process need strict temperature control and need hazardous chemicals. So, it is risk to azoic dyeing at home.