Introduction:
A natural dye-bearing material contains only a small amount of colouring matter or dye in addition to other plant and animal constituents such as water-insoluble fibres, carbohydrates, proteins, chlorophyll, and tannins, among others, and extraction is therefore essential to the preparation of pure natural dyes as well as to the use of crude dye-bearing materials. As raw colouring materials are not a single chemical entity and the plant matrix also contains a variety of non-dye plant constituents so that extraction is a complex process. Before utilizing an extraction process, it is necessary to determine the nature and solubility of the colouring materials.
You May Read: Fabric Dyeing with Beetroot: 4 Easy Step with Image
Natural Dye Extraction:
These different methods include:
- Aqueous extraction
- Alkali or acid extraction
- Microwave and ultrasonic assisted extraction
- Fermentation
- Enzymatic extraction
- Solvent extraction
- Super critical fluid extraction
You May Read: 4 Authentic Sources of Natural Dyes is Describe Easy Way
1. Aqueous extraction:
Traditionally, this method is used for extracting dyes from plants. It is a conventional method. In this method, the dye-containing material is first cut into small pieces and then dried until all moisture has been removed. When all moisture has been removed, the material becomes crispy. Blending the material into powder makes it powder. This mixture is then soaked in water in earthen, metal or wooden vessels. A copper or stainless-steel vessel is ideal. It is soaked overnight to loosen the cell structure. Following that, it is boiled to get the dye solution. It is then filtered to remove residual solids and the dye solution is ready. In the case of a large-scale extraction for purification of dye powder, stainless steel vessels are used, and boiling the solution for a prolonged period of time may reduce the amount of time needed to soak the materials in water. To separate residual matter, centrifuges are typically used. A trickling filter can ensure that fine plant material particles are removed and that the purified natural dye becomes more solubilized.
However, there are some drawbacks. They are as follows:
- Long time was required for this extraction process.
- There is a large amount of water needed for this process.
- The temperature needed to be high.
- Dye yield is low.
- Boiling reduces the yield of heat-sensitive dyes.
2. Alkali or acid extraction:
As many dyes are in the form of glycosides, they can be extracted under dilute acidic or alkaline conditions. By adding acid or alkali to glycosides, glycosides are hydrolyzed more efficiently, resulting in higher yields of coloring materials and better extraction. For the extraction of dye from tesu flowers (Butea monosperma), acidified water is used to prevent oxidative degradation of flavone dyes. Due to their soluble nature in alkali, phenolic dyes are suitable for alkaline extraction. Dyes can be precipitated with acids after alkaline extraction. This method is also used to extract lac dye from lac insect secretions and red dye from safflower petals. Dyes from annatto seeds can be extracted using this method. There are some disadvantage:
- After extraction, acid and alkaline are released into the environment. This affects the environment.
- Under acidic and alkaline conditions, some coloring materials may be destroyed.
- There are times when acid and alkaline extraction processes can adversely affect the fibers or materials being dyed.
You May Read: Latest Classification of Natural Dyes in 3 Way
3. Microwave and ultrasonic assisted extraction
Microwaves and ultrasound are used in these extraction processes to increase efficiency. The process reduces the amount of solvent required, the duration, and the temperature of extraction. The dye-containing materials are treated with water or any other solvent using this process. In the liquid, ultrasound forms small bubbles or cavitations. As they expand in size, they lose their shape after a certain length. When this happens, the bubbles burst, creating high pressure and temperature. Millions of bubbles form in a second, increasing the extraction efficiency quickly.
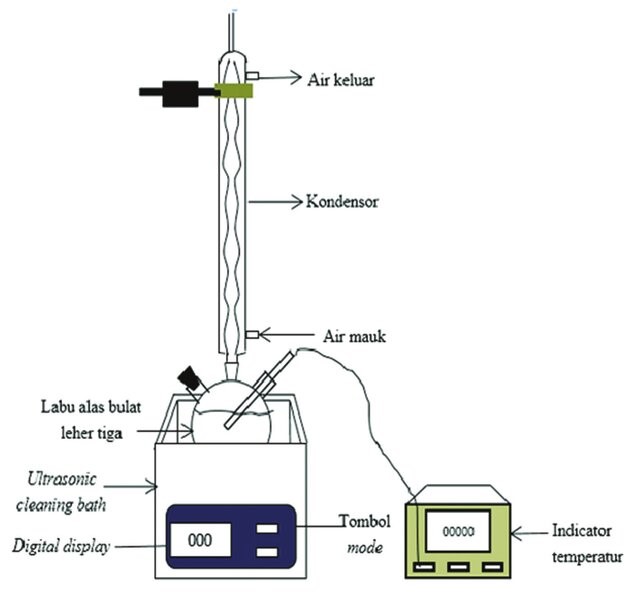
In microwave extraction, natural dyes containing minerals that are treated with a minimum amount of solvent in the presence of microwave energy sources. Microwaves speed up the extraction process and increase yield.
Microwave and ultrasound extractions are considered green processes since they use low temperatures, solvents, and time, consuming less energy. However, they are expensive process.
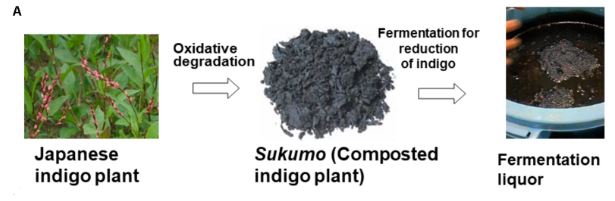
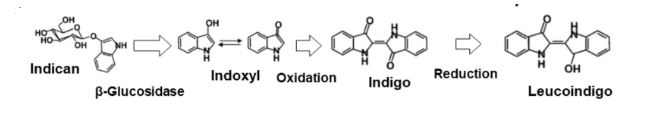
4. Fermentation:
A fermentation extraction method uses enzymes produced by microbes. The whole process takes 10-15 hours. Indigo dye extraction is the most common example of a fermentation extraction process. Freshly harvested indigo leaves are soaked in warm water at 32 C . Indimulsin enzyme breaks down colorless indigo containing glucoside indican and transforms it into glucose and indoxyl after 10-15 hours. There is also an enzyme called Indimulsin in indigo dye leaves. After 10-15 hours, yellow liquor containing indoxyl is taken to beating vats. In the beating vats, indoxyl gets oxidized by the air to become blue. After removing all excess water, found indigo dyes in cake form. But there are some disadvantages. They are:
- The extraction process takes a long time.
- After harvesting, pigments must be extracted immediately.
- As a result of microbial action, there is a foul smell.
5. Enzymatic:
As plant tissues contain cellulose, starches, and pectins as binding materials, some researchers have used commercially available enzymes like cellulase, amylase, and pectinase to loosen the surrounding material, allowing dye molecules to be extracted under milder conditions. Plant bark, roots, and the like can be used to extract dyes through this process.
6. Solvent extraction:
Organic solvents such as methanol, acetone, chloroform, petroleum ether, and mixtures of solvents such as ethanol and methanol or water with alcohol can be used to extract natural dyes. A water and alcohol extraction method can be used to extract dyes that are both water-soluble and water-insoluble. The extraction yield is therefore higher compared to aqueous extraction because a larger number of chemicals and coloring materials can be extracted. In addition to adding acid or alkali to alcoholic solvents, coloring matter can also be released by hydrolyzing glycosides. It is easier to purify extracted colors since solvents can be removed and reused through distillation. Extraction is done at a lower temperature, so degradation is less likely. There are some disadvantage:
- Toxic residual solvents may contribute to greenhouse gas emissions.
- Because the extracted material is not readily soluble in water, the dyeing process must be carried out in an aqueous medium.
- There are also problems associated with coextraction of substances such as chlorophylls and waxy materials.
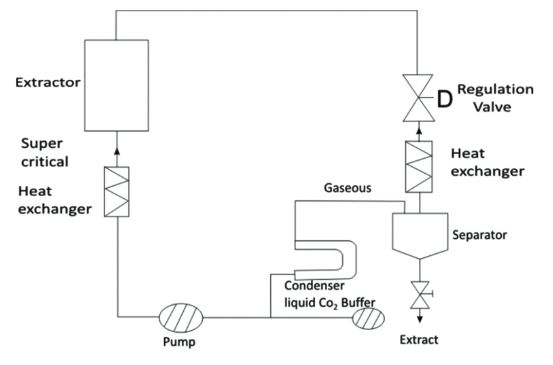
7. Super critical fluid extraction
The extraction of natural products with supercritical fluids is a new field. Gases function as supercritical fluids above their critical temperatures and pressures. A fluid of this type has properties somewhere between liquid and gas. Because they have much lower surface tension than liquids, they can spread out more easily along a surface. The low viscosity also makes them very diffusible, allowing them to interact better with the substrate. As solubility in any solvent increases at higher pressures and temperatures, a supercritical fluid is able to dissolve many substances like a liquid, so such conditions are necessary to maintain a gas in a supercritical state. A supercritical fluid extraction using carbon dioxide (CO2) is a good substitute for solvent extraction because it is cheap, easy to obtain, and does not leave residues. Carbon dioxide has critical pressure and temperature values of 1,070 pounds per square inch and 73.8 bars, respectively. Temperatures and pressures typically range between 32 and 49 °C when supercritical CO2 is used. Considering that CO2 is a nonpolar molecule, it behaves like a nonpolar organic solvent. To make slightly polar solutes more solubilized, co-solvents or modifiers can be added. As the process does not contain residual solvent traces or heavy metals and is light colored due to the absence of polar polymerizing substances, it has gained popularity as a method of obtaining pure natural products suitable for food and pharmaceutical use. Disadvantage of these method:
- It is an expensive method because expensive equipment is required.
- The extraction of polar substances is poor.
Reference:
- Sinha K, Chowdhury S, Saha PD, Datta S (2013) Modelling of microwave-assisted extraction of natural dye from seeds of Bixa orellana (Annatto) using response surface methodology (RSM) and artificial neural network (ANN). Ind Crops Prod 41:165–171
- Kasiri, M. B., & Safapour, S. (2014). Natural dyes and antimicrobials for green treatment of textiles . Environmental Chemistry Letters, 12(1), 1–13. https://doi.org/10.1007/s10311-013-0426-2
- Raja, S. S. and A. S. M. (2014). Natural Dyes: Sources, Chemistry, Application and Sustainability Issues. Textile Science and Clothing Technology, 37–80. https://doi.org/0.1007/978-981-287-065-0_2
- Salauddin Sk, M., Mia, R., Haque, M. A., & Shamim, A. M. (2021). Review on Extraction and Application of Natural Dyes. Textile & Leather Review, 6257(June), 1–16. https://doi.org/10.31881/TLR.2021.09
- Aino, K., Hirota, K., Okamoto, T., Tu, Z., Matsuyama, H., & Yumoto, I. (2018). Microbial communities associated with indigo fermentation that thrive in anaerobic alkaline environments. Frontiers in Microbiology, 9(SEP), 1–16. https://doi.org/10.3389/fmicb.2018.02196
- Fisher El, Otto M, C. G. (2018). Adapted from Basis of virulence in enterotoxin-mediated staphylococcal food poisoning b. Frontiers in Microbiology, 9, 436.