Best 15 Differences Between silk and Wool
Introduction
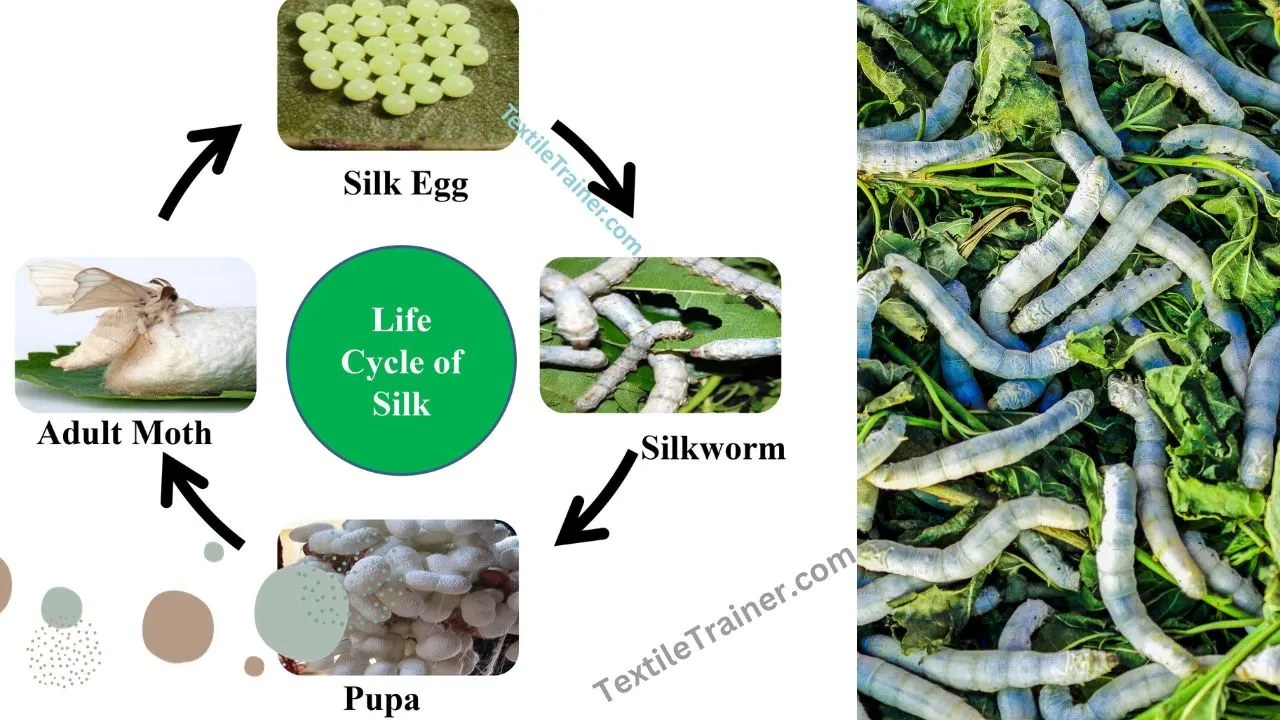
Silk and Wool both are natural protein fiber. But they have some differences. Silk is a natural fiber that is extracted from an animal source. Some forms of silk can be used as clothing. It is made from silkworm cocoons. Although several insects produce silk, it is only the moth caterpillar’s silk that is used for textile production. The most popular form of silk is obtained from the cocoons of the mulberry silkworm Bombyx mori.
It can be used to make fabrics such as chiffon, charmeuse, crepe de chine, taffeta, habutai, and tussah. On the other hand, wool is also natural fiber. In textiles, wool is a kind of fiber obtained from furry animals, like sheep. Different kinds of wool are available, such as cashmere, mohair, and angora.

Difference between silk and wool
| S/N | Silk | Wool |
| 01. | Silk is produced from the cocoons of silkworms. | Wool is produced from fur of animals. |
| 02. | These fiber consist oxygen, hydrogen, nitrogen and carbon. | These fiber consist of hydrogen, nitrogen, carbon and sulfur. |
| 03. | Crystallinity of these fiber is higher than wool. | Crystallinity of these is less than silk. |
| 04. | It is more sensitive to heat. | It is less sensitive to heat. |
| 05. | It has less insulating power. It is not as effective as the wool in keeping body warm. | It has has a good insulating power. |
| 06. | As, these fiber has less amorphous area, absorbency of moisture is less than wool. | As, these fiber has much amorphous area than silk, it has good absorbency power. |
| 07. | Silk doesn’t contain disulphide bonds. | Wool contain disulphide bonds i.e. cysteine. |
| 08. | Attractions between silk polymers are thought to be hydrogen bonds. | Attractions between wool polymers are thought to be hydrogen bonds and van der waals forces. |
| 09. | Silk polymer is composed of sixteen different amino acids. | Wool polymer is composed of twenty different amino aicds. |
| 10. | Silk polymer is about as long as 140 nm, 0.9 nm thick. | Wool polymer is shorter than the silk polymer. |
| 11. | Chemical bonding group of silk polymer are carboxyl, hydrogen and amino group. | Chemical bonding group of wool polymer are disulfide bonds, hydrogen bonds, salt linkages, and peptide etc. |
| 12. | Continuous exposure to light weakens silk faster than wool. | it has well resistant against sun light. |
| 13. | Due to its extended polypeptide chains, it is less elastic and resilient. | A folded polypeptide chain makes it more elastic and resilient. |
| 14. | Any time of year can use for silk clothes. | In winter, woolen clothing is usually worn. |
| 15. | It has a shimmery and shiny appearance. | It doesn’t have a shiny appearance. |
Why silk polymer is different from wool polymer?
The polymer of silk differs from the wool polymers as follows:
- The silk polymer compose of sixteen amino acids as opposed to the twenty amino acids in the wool polymer. Three of these amino acids constitute about four-fifths of the silk polymer.
- Silk polymers do not contain any amino acids containing sulfur. As a result, silk polymers do not contain any disulphide bonds.
Identification of Silk
Identification of silk is A burn test is the best way to tell whether silk or wool is the same. Silk leaves powdery ash when burned and extinguishes when it is removed. The most easy way to distinguish between the two is their smell. Silk will have a much more disagreeable odor than wool, which smells like burning hair.
- Burning test: The smell of burning silk is similar to that of horns or hair.
- Combustion: A small flame that slowly extinguishes itself.
- Residue: Black, friable cinders and weighted silk leave a crystallized ash.
Identification of Wool
- Physical test: the flame is steady but more difficult to keep buring. the smell of burning wool is like burning of hair.
- Chemical test: wool fiber dissolves in concentrated sodium hydro-oxide and sodium hypo-chloride and slowly dissolves in Nitric acid 70%

Distinguishing Wool from Silk
To distinguish wool fiber from silk, cold hydrochloric acid and sodium hydro-oxide solution are used. At first, wool and silk fiber are treated with concentrated cold hydrochloric acid and sodium hydro-oxide solution. After sometimes, we will see silk fiber dissolve but the wool fiber swells.
You May Read More:
- Intriguing History of Silk Discovery with Geographical Distribution
- 6 Easy Step of Silk Manufacturing Process
- Source of natural fiber.
- Simple Life Cycle of Silkworm with 4 Easy Stage
- Best 10 Different Types of Silk in the World
- Macro and Micro Structure of Silk Polymer
- 6 Physical and Chemical Special Properties of Silk: Ensure High Quality
- Best 15 Differences Between silk and Wool
- 4 Special End uses of Silk Fabric: Luxury Dress for wedding