Name of the Experiment:
Prepare a lab report on bleaching of cotton fabric
Introduction:
Bleaching is the process of removing the natural color of textile fibers in order to achieve whiteness so that true colors can be developed during coloration. As a result of the oxidation process, the coloring maters are destroyed; dirt or even coloring matters cannot be removed. Although it is not mandatory in dark shades, it is absolutely necessary for pale and brilliant shades, and modified light bleach is sufficient in medium shades. There are several ways to bleach, that is bio-bleaching or chemical bleaching. Chemical bleaching is still very popular because of its good bleaching results. Almost all bleaching agents negatively affect fiber, so the process should be done as quickly as possible with the least amount of damage. Cotton fiber can be degraded by strong and uncontrolled bleaching actions; therefore, the process must be carefully regulated.
Objectives:
- To know about bleaching process.
- To know recipe calculation of bleaching process.
- To know working procedure of bleaching process.
The following oxidative bleaching processes are suitable for textile processing:
- Hydrogen per-oxide (H2O2) bleaching.
- Bleaching powder [ Ca(OCl)Cl]
- Sodium hypochlorite (NaOCl) bleaching.
- Sodium Chlorite (NaClO2) bleaching
In this experiment , we will used hydrogen per-oxide bleaching agent for bleaching process of cotton fabric.
Textile goods are bleached most frequently using hydrogen peroxide bleaching, which has a high and stable degree of whiteness. An organic peroxide stabilizer is used to stabilize the bleaching bath. Stabilizing the bleaching bath depends on water hardness; fluctuations in the water harness are not significant when the stabilizer is used. It is important to prevent catalytic damage to the goods by using water that does not contain heavy metal ions and metal accessories on the goods. In order to achieve successful bleaching, the bath stability must be optimal. Hydrogen peroxide bleach solution stability depends on several factors, including:
- pH: During bleaching, hydrogen peroxide should be maintained at a constant pH (not too high) as it is stable in acidic solution and unstable in alkaline solution.
- Temperature: At higher temperatures, hydrogen peroxide solution becomes unstable and decomposes faster.
- Buffers: A mixture of silicates, phosphates, borax, and proteins stabilizes the hydrogen peroxide bath.
- Hard water: Depending on the hardness of the water and the metals causing it to become hard, peroxide can be stable or unstable.
- Metals: In the presence of silicates, Ca and Mg tend to stabilize the hydrogen peroxide bleach solution; Cu, Fe, etc. tend to un-stabilize it.
- Peroxide concentration: To prevent fiber decomposition, it is necessary to maintain a constant concentration of peroxide.
Mechanism of Bleaching (H2O2):
An effective bleaching agent, hydrogen peroxide can be used for wool, silk, cotton as well as union fabrics that contain cotton, wool, or silk. It is an excellent bleaching agent for fabrics made of cotton, silk, and wool; it can even be used for sheet fabrics.
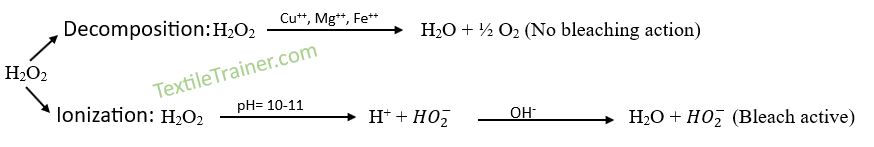
Hydrogen peroxide is stable in acid medium. if we add alkali, it produced per-hydroxyl (HO2–) ion which is responsible for bleaching action. but presents of Cu++, Mg++, Fe++ ion, decompose the hydrogen peroxide and produce oxygen which have no bleaching power. That’s why we add NaOH which is help to ionization of hydrogen peroxide and produce per-hydroxyl (HO2–) ion which have bleaching action.
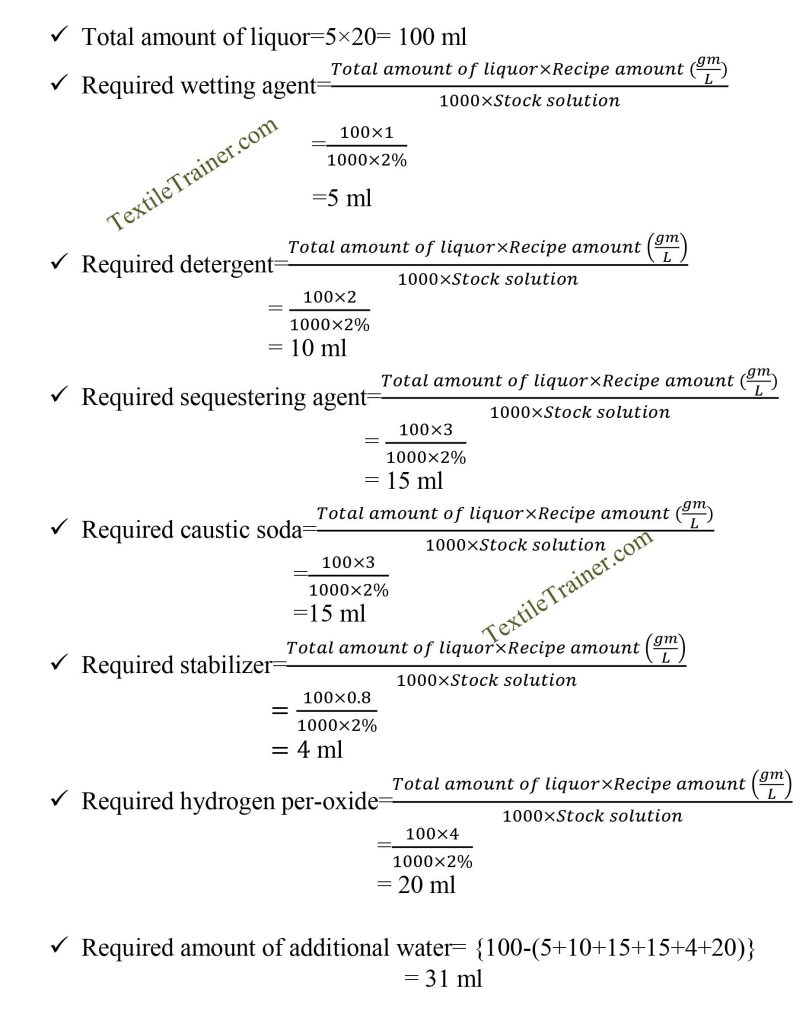
Typical Recipe for bleaching of Cotton Fabric
| Chemical | Amount (gm/l) |
| Wetting agent | 0.5-1.0 gm/l |
| Sequestering agent | 1.0-3.0 gm/l |
| Detergent | 1.0-2.0gm/l |
| Stabilizer | 0.3-0.8 gm/l |
| Caustic soda | 1.0-3.0 gm/l |
| Hydrogen peroxide | 4.0-8.0 gm/l |
| Temperature | 90-950 C |
| Time | 20-45 min |
| pH | 10-11 |
| M:L | 1:20 |
| Sample weight | 5 gm |
Recipe Calculation:
Process Curve:
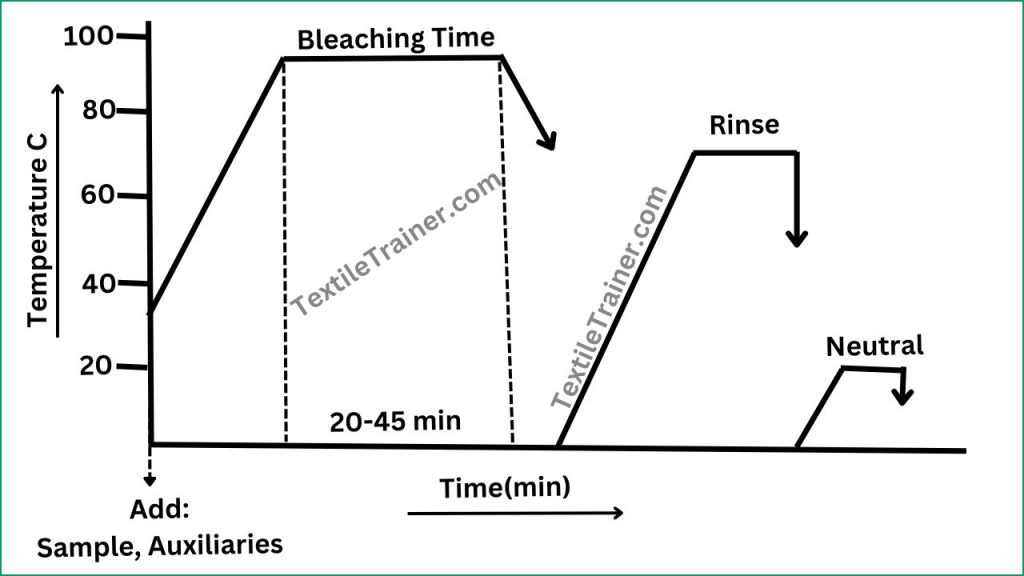
Working Procedure:
- Set the bleaching bath with sample with auxiliary agent, stabilizer and caustic soda at room temperature.
- Now rise the temperature and add hydrogen per-oxide after 5 min.
- Then rise the temperature to 90-950C.
- For rapid bleaching need higher temperature at lower time. Run the bath for 20-45 min. this time calculate as bleaching time.
- After 20-45 min, cool down to 700 C and drop the bath.
- Now per-oxide killer is applied to remove residual per-oxide from the sample.
- Then neutral the sample.
Result:

Conclusion:
In order to dye natural fiber, the bleaching process is one of the most crucial stages of dyeing. During this experiment, we learned about the bleaching process and also how to calculate a recipe for the bleaching process. However, we learned that this experiment would be useful in the future. Thanks to our teacher for helping us with this experiment.







2 thoughts on “Study on Bleaching of Cotton Fabric with Easy Recipe Calculation/Lab Report-5”