What is pH Meter?
An instrument that is used to measure hydrogen ion [H+] activity in solutions is called pH meter. pH is based on the ratio of hydrogen ion [H+] concentrations to hydroxyl ion [OH+] concentrations which is determine the level of hydrogen ion [H+] activity in a solution. In this article, we will discuss how to calibrate pH meter simply.
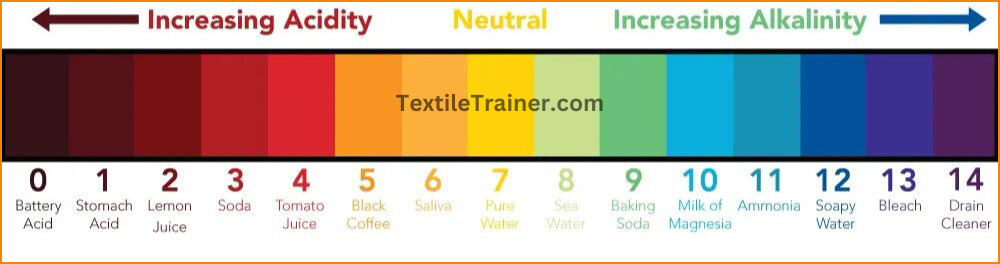
pH Scale:
This pH level generally ranges from 1 to 14. Following described the parameter of pH scale.
- pH is 7 for neutral solution. ( Here hydrogen ion and hydroxyl ion has same activity)
- pH is less than 7 for acidic solution. ( Here hydrogen ions activity is greater than hydroxyl
- pH is greater than 7 for alkaline solution. (Here hydroxyl ions activity is greater than hydrogen ion).
Why Calibrate pH Meter??
It is important to calibrate your pH meter regular basis. But it depends on its usage. To avoid measuring errors, we should ensure our pH meter is calibrated two times a month. In the following cases, it is recommended to calibrate the pH meter:
- Before use new electrode.
- The electrode hasn’t been used for a long time or period.
- After electrode has been cleaned.
- We have to calibrate pH meter after measuring a strong solution.
Required Chemical for Calibration pH Meter:
Two chemical is required to calibration pH meter. They are:
- Buffer solution pH-4
- Buffer solution pH-7
Calibration Procedure:
- To calibration of the pH meter, at first we place the electrode of the pH meter in the distilled water. Distilled water pH is 7. But our pH meter indicate 8.1. So our pH meter needs to calibration.
- Now, we took ethanol in beaker to clean the electrode of the meter.
- Then, we took buffer solution pH-7 in a beaker. Dipped the tip of the electrode in the buffer solution. We will wait until the reading stable. pH meter indicated that 7.4. Now, we used small screwdriver to adjust the pH-7 trimmer until display reading pH-7.
- Again, we used ethanol to clean the electrode.
- Then, we took buffer solution pH-4 in a beaker. Dipped the tip of the electrode in the buffer solution. We will wait until the reading stable. pH meter indicated that 3.9. Now, we used small screwdriver to adjust the pH-4 trimmer until display reading pH-4.
- Now, let’s check our meter. We dipped the electrode in distilled water. pH meter indicate pH-7.
- So, our calibration process is completed.
You Can Watch This Video
How To Make pH Meters More Accurate?
You can ensure that your pH meter readings are more accurate by cleaning, storing, and calibrating it properly.
- Store the pH meter according to the manufacturer’s instructions.
- The glass membrane of the sensor bulb can be damaged if you wipe it after rinsing it.
- Cover the pH meter’s sensor bulb with a rubber cap and filled with pH Electrode fill solution 4M KCl if you are storing it for a long time.
- Maintain a regular electrolyte level inside the electrode to prevent a dried-out junction.
- When measuring pH, make sure the temperature does not fluctuate.
Reference:
- Classification of Mordant with 3 Simple Mordanting Method of Natural Dye.
- Natural Dye Extraction Process: 7 Easy Technique.
- Fabric Dyeing with Beetroot: 4 Easy Step with Image.
- 4 Authentic Sources of Natural Dyes is Describe Easy Way.
- Latest Classification of Natural Dyes in 3 Way.
- Latest History of Knitting Technology Development: Early to Modern.
- 3 Easy Step of Non-woven Fabric Manufacturing Process with Modern Application.
- Best 4 Types of Composite Test for Smart and Sustainable Composite.
- Is it Necessary to Sizing of Filament Yarn or Synthetic Yarn? Dynamic Explain.
- 15 Characteristics of Good Size Ingredients and Factors of Choose Size Ingredients.






